Approval of Leqembi comes after controversial approval in 2021 of Aduhelm, which met with criticism over concerns about that drug’s effectiveness, safety, pricing
By Physician’s Briefing Staff HealthDay Reporter
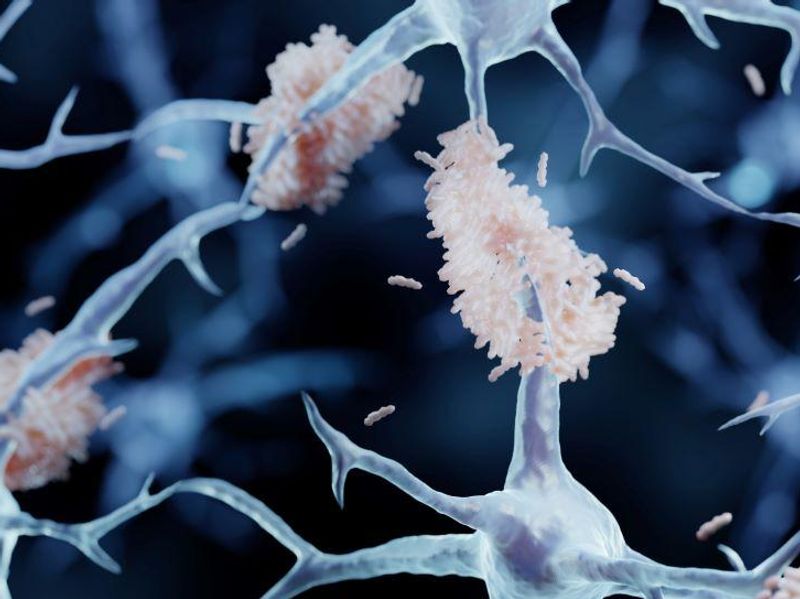
FRIDAY, Jan. 6, 2023 (HealthDay News) — The U.S. Food and Drug Administration on Friday approved a second drug for Alzheimer disease, Leqembi (lecanemab-irmb), despite reports of rare brain bleeds linked to use of the drug in some patients.
Leqembi, made by Eisai and marketed by Biogen, will be only the second drug for Alzheimer disease to receive FDA approval in the past 18 months; the agency’s speedy approval of the drug Aduhelm in June 2021 generated controversy in the medical community over its lack of effectiveness, brain bleed concerns, and hefty price tag.
And not every patient would stand to benefit from Leqembi, stressed the Babak Tousi, M.D., from the Cleveland Clinic. He led the portion of the clinical trial that was conducted at the Cleveland Clinic. “The trial was designed for patients in the earlier stage of Alzheimer’s disease, people with mild cognitive impairment or early stage of dementia,” Tousi noted.
The results of the 18-month trial, which involved about 1,800 patients, gained wide attention when they were published late last year in the New England Journal of Medicine, Tousi noted. In the trial, early-stage Alzheimer disease patients who took Leqembi showed a 27 percent reduction in their mental decline compared with patients in the placebo arm of the trial. The drug’s users also showed less evidence of amyloid protein plaques in their brain compared with nonusers.
Still, the deaths of two patients enrolled in the trial cast a cloud on these hopeful findings. Both died from brain hemorrhages that seem linked to the use of Leqembi.
A 65-year-old woman with early-stage Alzheimer disease recently died from a massive brain bleed that some researchers link to lecanemab, according to a report published Nov. 27 in ScienceInsider. The woman suffered a stroke as well as a type of brain swelling and bleeding that has been previously seen with such antibodies, the report noted. Emergency physicians at Northwestern University Medical Center in Chicago treated the woman with a tissue plasminogen activator. She immediately had substantial bleeding throughout her brain’s outer layer. The woman died a few days later, according to the case report. The death follows that of an 80-year-old man who was taking part in lecanemab’s phase 3 clinical trial. His death was linked to a possible interaction between the experimental drug and the blood thinner apixaban.
The clinical trial also showed that 2.8 percent of participants who took the drug had a symptomatic side effect called amyloid-related imaging abnormalities (ARIA), which involves swelling in the brain. ARIA was not seen among any participants who got the placebo.
“Lecanemab clearly did what it was designed to do — it removed amyloid plaque,” Tousi, who heads the Clinical Trials Program at the Cleveland Clinic Center for Brain Health, told HealthDay. “The results demonstrated all the downstream effects we hoped would happen in terms of reduction of biomarkers and less clinical decline on several functional and cognitive measures. So, this difference will likely translate to a longer period of independent living for patients.”
Copyright © 2022 HealthDay. All rights reserved.